Optogenetics: Light as method for brain cell remote control
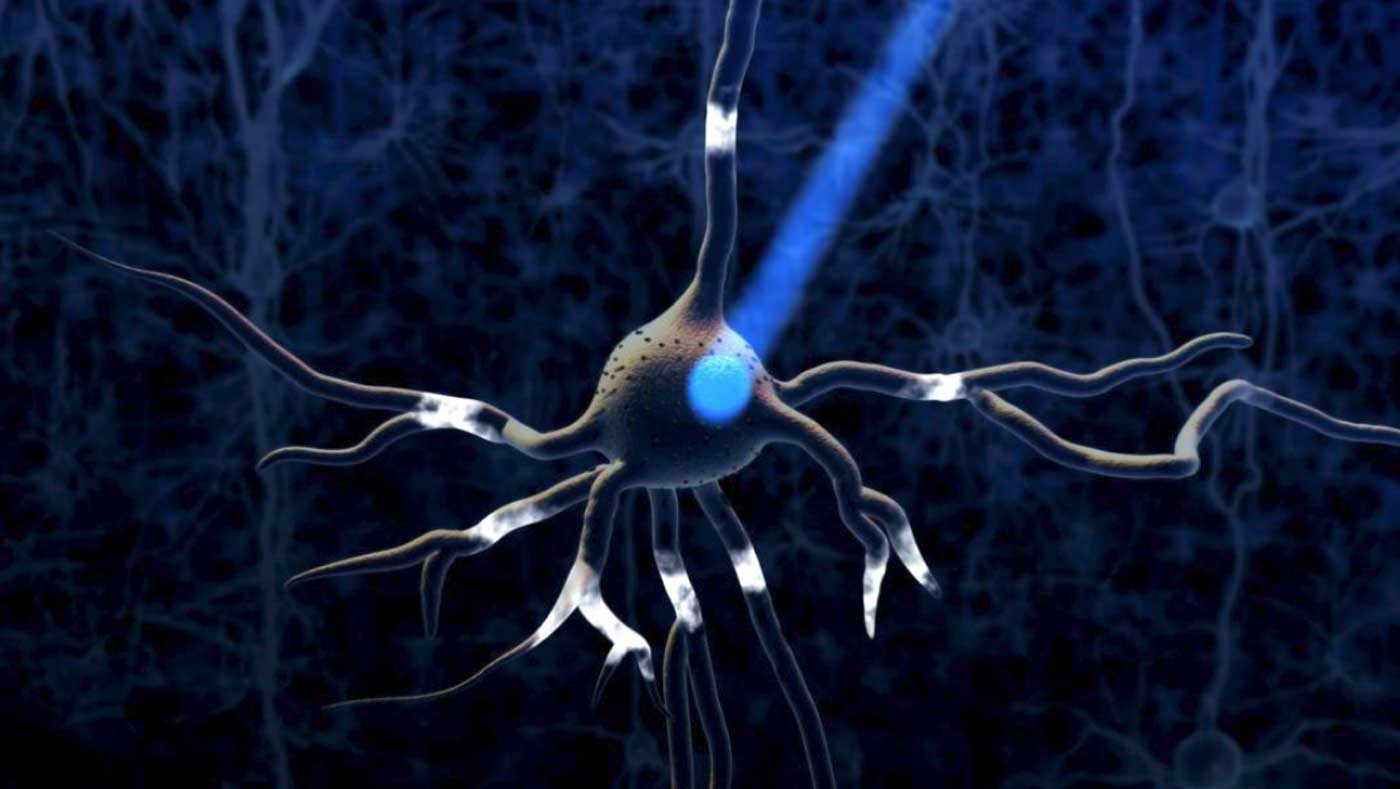
WHAT IS OPTOGENETICS?
Optogenetics (from Greek optikós 'seen, visible') most commonly refers to a biological technique that involves the use of light to control neurons that have been genetically modified to express light-sensitive ion channels. Optogenetics is a method for controlling a neuron’s activity using light and genetic engineering.
Most all cells in the body maintain a charge gradient (an imbalance in the concentration of positively and negatively charged ions) across their cell membranes. This gradient creates a polarized electric potential, or voltage. Neurons are one of the few cell types that are electrically excitable, meaning that when enough ions cross the membrane to reduce the ion imbalance to a specific threshold, the neuron fires. As a result, the cell rapidly opens ion channels and allows ions to rush inside that reverse the charge gradient.
When a neuron fires, the electrical signal is propagated down a long cellular extension called an axon to the axon terminal, where a neuron chemically communicates with its neighbor by releasing chemicals called neurotransmitters into a small space between the two cells, called the synapse. The neurotransmitters consequently cause ion channels to open in the neighboring neuron and the process repeats itself, transmitting the signal down a chain of neurons.
Optogenetics uses special ion channels that open and allow ion passage upon exposure to light of a specific color. These channels are not present in most multicellular organisms, but can be added to these animals’ cells using genetic engineering. Unlike previous techniques of stimulating neurons, which used electrodes that indiscriminately shocked neurons into firing, optogenetics allows precise activation of the specific cell types into which the ion channels are introduced.
The process of changing the information in the genetic code (the blueprints) of a living thing by adding or deleting information. Genetic engineering is sometimes called genetic modification.. Genetic engineering is a process where scientists change the information in the genetic code (the blueprints) of a living thing. In optogenetic studies, scientists take the genetic code of the neurons they want to study and add a new piece of code to it. The new code allows these neurons to make special proteins, called opsins. Proteins that respond to a specific type of light (for example, ChR2 only responds to blue light). In neuroscience, these proteins are used to control neuron activity., which respond to light. Opsins occur naturally and were first discovered in algae, which use these proteins to help them move toward light.
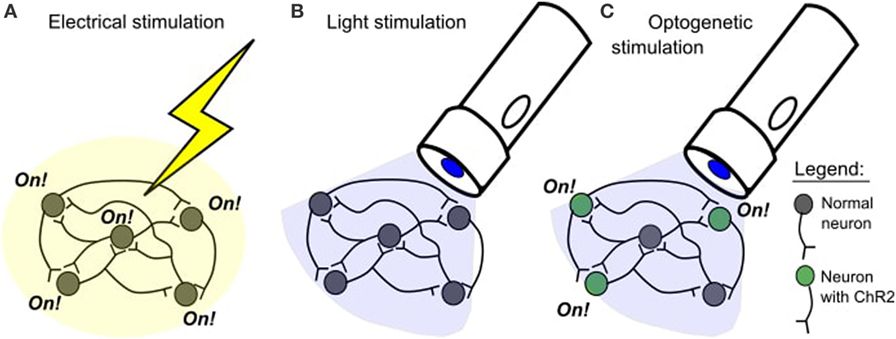
In neuroscience, the most popular opsin is called channelrhodopsin-2 (ChR2)
Opsins are a group of light-sensitive 35–55 kDa membrane-bound G protein-coupled receptors of the retinylidene protein family found in photoreceptor cells of the retina. Five classical groups of opsins are involved in vision, mediating the conversion of a photon of light into an electrochemical signal, the first step in the visual transduction cascade.
Scientists have created a light-responsive opsin so sensitive that even when engineered into cells deep within tissue it can respond to an external light stimulus.. Experiments in mice and macaques showed that shining blue light on the surface of the skull or brain was sufficient to activate opsin-expressing neurons six millimeters deep.
“I was pretty blown away that this was even possible,” says Gregory Corder, who studies the neurological basis of pain and addiction at the University of Pennsylvania and who was not involved with the work. At that sort of depth, he continues, “essentially no part of the rodent brain is off-limits now for doing this non-invasive [technique]. . . . It’s pretty impressive.”
“This development will help to extend the use of optogenetics in non-human primate models, and bring the techniques closer to clinical application in humans,” adds neurological disease expert Adriana Galvan of Yerkes National Primate Research Center in an email to The Scientist. Galvan was not a member of the research team.
Experiments in mice showed that when the novel opsin—which the authors name SOUL, for step-function opsin with ultra-high light sensitivity—was produced in cells anywhere in the brain, blue light from a fiber optic cable placed against the outside of the skull was sufficient to activate the neurons. Indeed, activation of SOUL-producing neurons in the lateral hypothalamus, approximately 5.5–6.2 mm below the skull surface, inhibited feeding behavior in hungry mice—a known effect of stimulating this region.
References:
---
1) https://kids.frontiersin.org/article/10.3389/frym.2017.00051
2) https://www.genenames.org/data/genegroup/#!/group/215
3) https://www.the-scientist.com/news-opinion/deep-brain-optogenetic-control-without-implants-67498
4) https://en.wikipedia.org/wiki/Optogenetics#:~:text=Optogenetics (from%20Greek%20optik%C3%B3s%20'seen,express%20light%2Dsensitive%20ion%20channels.
5) http://sitn.hms.harvard.edu/flash/2014/total-recall-using-light-to-create-and-erase-memories/





